An old Dutch saying is: Lime makes rich farmers and poor sons.
Ca forces +ions of the clay/humus complex, that gives a soil that is empty.
(except for Ca)
Balance is the Key!
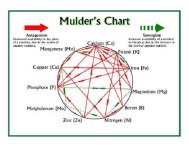
The most graphic illustration of the interaction of essential elements is called a Mulders Chart (Figure 1). This chart demonstrates the effect that some elements have on the availability of others to the plant. Some elements work in synergy — they stimulate the uptake of others and increase their availability. Some elements are antagonistic — they interfere with the uptake or availability of others. In addition, excessive applications of some elements can result in binding with others, causing lockups and making the latter unavailable; for example, excess liming (calcium) affects the availability of magnesium, zinc, iron, potassium, and manganese.
It’s important to understand the influence of balanced nutrition. Two plants can be grown at the same time yet yield different results. One might be grown in sand and one in a good garden loam. Both are planted at the same time. The end result might be one is small because it had a limited supply of nutrients and other large because it had a good supply, both plants, though will be healthy and normal because they had a balanced supply of nutrients. Health and normality can be present without optimal development having taken place. It’s all a matter of what you put into the plant and how you do it.
In short, you can use all the N-P-K you want, you will see the plant “grow” but this has little to do with plant health. To me, it is absurd to even consider reproducing nature’s food for healthy plants solely in a lab, when the world’s leading botanists tell us how little we know about how plants actually grow. This is why, even with all of our fertilizer programs, we always include natural inputs with any chemical inputs: humates, fish and seaweed, and carbon, and those that support soil fertility, microbial activity and plant health and that buffer chemical inputs. Consider chemical inputs as tools that you can learn to use to enhance your situation and profitability, providing you understand the correct way to use them.
Other practices like soil testing, plant tissue testing, crop rotation, microbe counts, and continual education all contribute to building a long-term sustainable program, and I encourage growers to consider these on an ongoing basis. People, like home gardeners, who may not do soil or tissue testing, cannot possibly know, or be expected to know, what specific elements are required and in what ratios. They are exposed to a huge range of products in pretty packets, so they continue to, collectively, spend billions of dollars on products they do not completely understand.
Conclusion
So, balance is the key. Prior to applying chemical fertilizers or biocides, ask yourself, “Will nature approve?” Does it make sense to continually use any single product year after year, or continually spray toxic chemicals because of constant bug or disease infestations? Given that fungal spores latch onto the surface of weak plants, would it not make sense to strengthen the plant instead of continually spraying fungicides?
If your situation does not include or warrant proper test procedures, find out about what you are using and question whether it contributes or detracts from soil and plant health long term. At the end of the day, whatever we put on our soil ends up in our mouths. And given that in Australia (and I suspect in North America, too) degenerative diseases like hearth disease, cancer, and obesity have dramatically increased over the last 100 years, shouldn’t we look at what we were eating 100 years ago as an indicator or guide to better health? Are we using chemicals as a tool or are they dictating our habits? From my experience, when plants with good nutrition are challenged, they generally recover.
https://sp.yimg.com/ib/th?id=HN.608011780074964064&pid=15.1&P=0